Enzymes
7.6.1 State that metabolic pathways consist of chains and cycles of enzyme- catalysed reactions.
Metabolic pathways consist of chains and cycles of enzyme-catalysed reactions.
7.6.2 Describe the induced-fit model.
Initially the substrate does not fit perfectly into the active site of the enzyme. When the substrate binds to the active site, this changes the shape of the active site and only then does it perfectly fit the substrate. As the substrate binds it changes the shape of the active site and this weakens the bonds in the substrate and therefore reduces the activation energy. This model is a more precise version of the lock and key one. The reason for this is that it explains why some enzymes can bind to many different substrates. If the shape of the active site changes when a substrate binds, this allows many different but similar substrates to bind to the one enzyme.
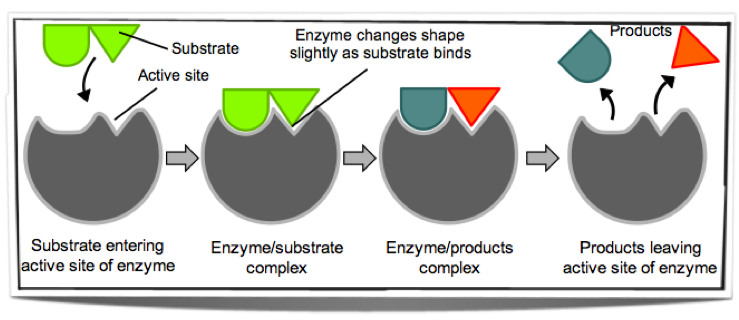
Figure 7.6.1 - The induced-fit model
7.6.3 Explain that enzymes lower the activation energy of the chemical reactions that they catalyse.
Reactants of a chemical reaction need to gain energy before they can undergo the reaction. This required energy is called the activation energy of the reaction and it is needed to break bonds within the reactants. At a later stage in the reaction energy will be released as new bonds form. The majority of biological reactions are exothermic. In exothermic reactions the energy released by the new bonds formed is greater than the activation energy. In other words, the reaction releases energy. Enzymes make it easier for reactions to occur by decreasing the activation energy required in the reactions that they catalyse.
.png)
Figure 7.6.2 - Activation energy of an exothermic reaction
7.6.4 Explain the difference between competitive and non-competitive inhibition, with reference to one example of each.
Enzyme inhibitors are substances which inhibit enzyme activity. There are competitive enzyme inhibitors and non-competitive inhibitors.
Competitive Inhibitors
These are structurally similar to the substrate of the enzyme and bind to the active site. This means that when a competitive inhibitor binds to the active site of an enzyme, it prevents the substrate from binding to the active site. Only once the inhibitor has been released from the active site can the substrate bind. The inhibitor is called a competitive inhibitor as it competes with the substrate for the active site.
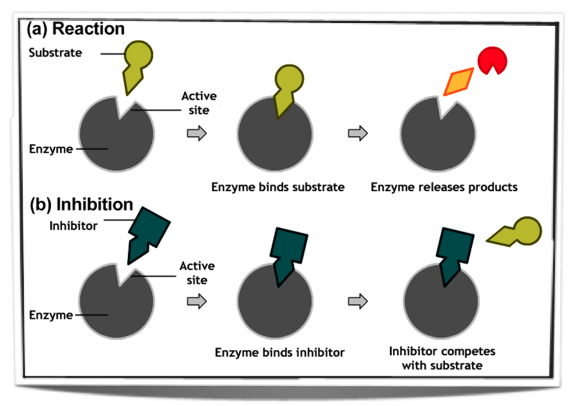
Figure 7.6.3 - Competitive inhibition
The effects of a competitive inhibitor can be reduced by increasing the substrate concentration. More substrate would successfully bind to the active site than inhibitor and therefore reducing the effect of the inhibition. The maximum rate of reaction or a level very close to the maximum rate of reaction can be reached.
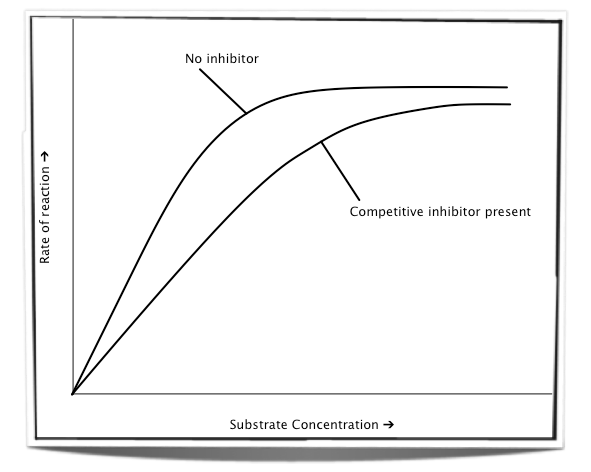
Figure 7.6.4 - The effect of increasing substrate concentration on a competitive inhibitor
An example of a competitive inhibitor is malonate. Malonate is structurally similar to the substrate succinate. Succinate is found in the Krebs cycle of aerobic respiration and binds to the active site of the dehydrogenase enzyme. Malonate can compete with succinate for the active site and in doing so it can prevent succinate from binding.
Non-Competitive Inhibition
These are not similar to the substrate and they do not bind to the active site of the enzyme. Instead they bind to a different site on the enzyme and change the conformation of the active site. The substrate may still be able to bind to the active site however the enzyme is not able to catalyse the reaction or can only do so at a slower rate.
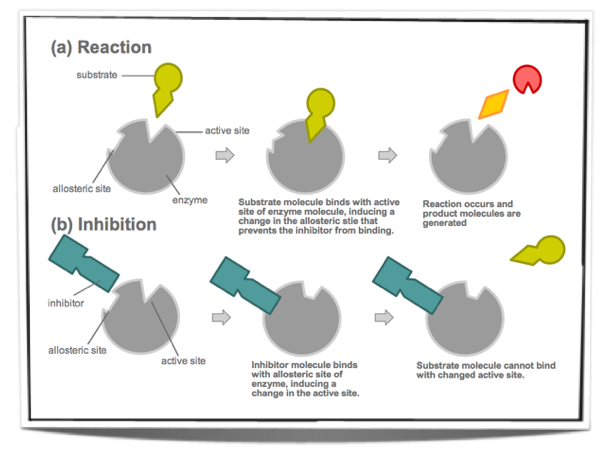
Figure 7.6.5 - Non-competitive inhibition
In the presence of a non-competitive inhibitor, increasing the substrate concentration cannot prevent the inhibitor from binding to the enzyme as the two bind to different sites. Therefore, no matter how high the concentration of substrate is, some of the enzymes will still be inhibited. The maximum rate of reaction will always be lower in the presence of a non-competitive inhibitor.
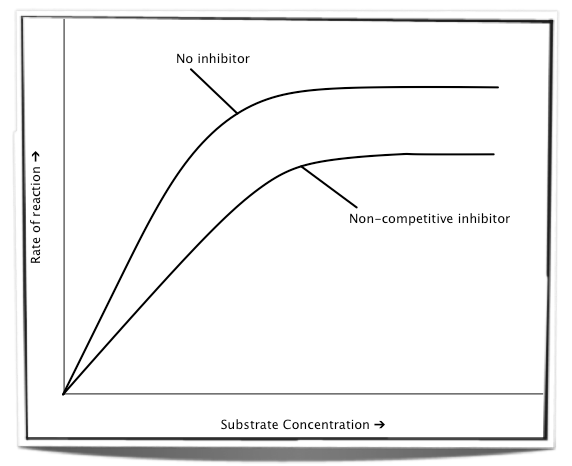
Figure 7.6.6 - The effect of increasing substrate concentration on a non-competitive inhibitor
An example of a non-competitive inhibitor is ATP. When ATP accumulates it binds to a site other than the active site on the enzyme phosphofructokinase. In doing so it changes the enzyme conformation and lowers the rate of reaction so that less ATP is produced.
7.6.5 Explain the control of metabolic pathways by end-product inhibition, including the role of allosteric sites.
Metabolic pathways are made up of many chemical reactions and these reactions are catalysed by enzymes. Often, the product of the last reaction in the pathway inhibits the enzyme that catalyses the first reaction of the pathway. This is called end-product inhibition and it involves non-competitive inhibitors.
The product of the last reaction of the metabolic pathway will bind to a site other than the active site of the enzyme that catalyses the first reaction. This site is called the allosteric site. When it binds to the allosteric site it acts as non-competitive inhibitor and changes the conformation of the active site. Therefore, it makes the binding of the substrate to the enzyme unlikely. Once the inhibitor is released from the allosteric site, the active site returns to its original conformation and the substrate is able to bind again.
There is a clear advantage in using end-product inhibition for controlling metabolic pathways. When there is an excess of end-product, the whole metabolic pathway is shut down as the end product inhibits the first enzyme of the pathway. Therefore less of the end product gets produced and by inhibiting the first enzyme it also prevents the formation of intermediates. When the levels of the end product decrease, the enzymes start to work again and the metabolic pathway is switched on.
